In recent years, functional soft candies have gained rapid popularity due to their “delicious taste and health benefits”. Data show that the global gummy market exceeded USD 8 billion in 2024 and is expected to surpass USD 16.7 billion by 2030, with mineral-based soft candies fortified with iron, zinc, and calcium emerging as the fastest-growing subcategory. As a new generation of mineral supplements, glycine-chelated mineral salts have been gaining favor in the market; it is projected that the global market size of such salts will reach USD 145 million by 2025 and increase to USD 265 million by 2035, representing a compound annual growth rate (CAGR) of 6.5%.
With high absorption efficiency, favorable palatability, and broad international compliance, glycine-chelated mineral salts have become the latest choice for brands to develop best-selling functional soft candies.
Global regulatory recognition, with applications continuing to expand:
In the United States, calcium, magnesium, ferrous, and zinc glycinates are classified as Old Dietary Ingredients (ODIs) and may be used in dietary supplements; ferrous bisglycinate has obtained FDA GRAS status.
In the European Union, glycinates passed EFSA evaluation in 2008; covered chelates include calcium, magnesium, iron, zinc, copper, and manganese for use in foods and dietary supplements.
In China, regulations are more granular: under GB 14880-2012, eligible applications include formulated milk powder, beverages, baked goods, and meat products. Relevant product standards include GB 30605–2014 (calcium glycinate), GB 30606–2014 (ferrous bisglycinate), and GB 1903.2–2015 (zinc glycinate).
At present in China, only ferrous glycinate is permitted for addition to gummies and other confectionery products; cross-border products are not subject to these specific restrictions.
As mineral supplements, absorption efficiency constitutes the core competitive advantage. The benefits of glycine-chelated mineral salts are foremost reflected in their absorption pathway: using iron as an example, ferrous iron (Fe²⁺) can enter the bloodstream directly via divalent metal transporter 1 (DMT1) on duodenal mucosal cells, yielding a short and efficient absorption route; by contrast, ferric iron (Fe³⁺) must first be reduced to Fe²⁺ before uptake, and this additional step results in an absorption rate only one-third that of ferrous iron [1]. The chelation of glycine with ferrous iron can further enhance bioavailability while simultaneously reducing gastrointestinal irritation [2].
Vitamin C: The “Absorption Accelerator” and “Flavor Guardian” of Ferrous Glycinate
Synergistic effects on absorption:
● Synergistic absorption: Co-administration with Vitamin C increases iron absorption 3.6-fold relative to iron alone [3].
● pH modulation: Vitamin C adjusts pH toward the optimal range for DMT1 (≈ 5.5). Lowering pH from 7.0 to 6.0 increases iron uptake 2–3×[4]. By donating protons in the intestinal lumen and acting in concert with Dcytb, Vitamin C creates the acidic microenvironment required by DMT1 [5], facilitating ferrous iron uptake.
● Oxidation control: In the gut, Fe²+ is readily oxidized to the less absorbable Fe³+. Vitamin C preferentially reacts with oxygen, limiting Fe²+oxidation; upon Vitamin C addition, the dissolved-oxygen decline rate increases ~30%.
● And Fe²⁺retention exceeds 90% [6].
Triple flavor modulation:
● Suppression of metallic notes: Inhibits the Fe²⁺→Fe³⁺ transition, thereby attenuating metallic taste and the formation of lipid oxidation products (e.g., hexanal).
● Acidity counterbalance: Vitamin C at pH 2.5–3.0 imparts a clean, refreshing acidity that effectively masks iron-related off-flavors.
● Enhanced fruity aroma: Co-formulation with fruit juice powders (e.g., jujube/red date) augments desirable aromatic notes and diminishes metallic off-notes.
Pure-Chel™ Glycinate: The “All-Rounder” for Gummy Applications
In gummies, the solubility and dispersibility of raw materials, as well as their sensory attributes, directly determine product competitiveness and influence texture uniformity, smoothness, and bioavailability. RICHEN Nutritionals’ Pure-Chel™ glycinates demonstrate significant advantages across these dimensions.
Pure-Chel™ Glycinates vs. Conventional Mineral Salts:
Owing to their chelated molecular structure, glycinates circumvent the grittiness, precipitation, and phase separation associated with traditional mineral forms (e.g., some sulfates and carbonates) that exhibit poor solubility or aggregation. This improves gummy smoothness and product stability. Beyond providing a crystal-free, non-precipitating mouthfeel, glycinates enable uniform distribution of actives and consistent per-piece potency, while offering complete dissolution and rapid absorption—substantially improving mineral utilization.
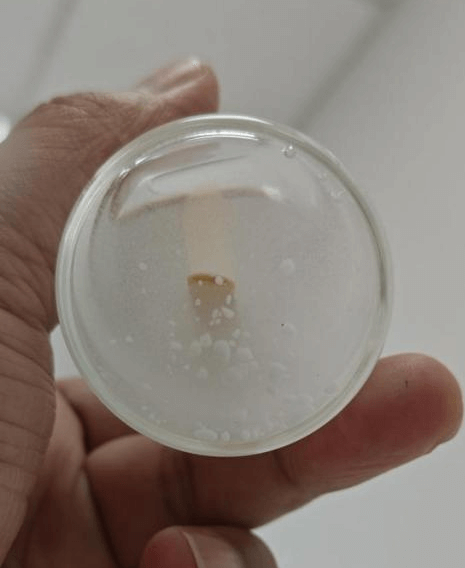
Pure-Chel™ Calcium Glycinate vs. Comparator Calcium Glycinate
In gummy formulation studies, the comparator calcium glycinate generated particulate matter within the syrup matrix, displayed limited solubility, and could not be incorporated as a post-cook addition, thereby constraining gummy manufacturing. By contrast, Pure-Chel™ calcium glycinate dissolved to an optically clear solution in the syrup system and remained free of visible particulates after several minutes of standing, identifying it as a superior choice for gummy applications.
Pure-Chel™ Magnesium Glycinate – Upgraded flavor Reduction technology
Palatability is the primary purchase driver for gummy consumers. E-commerce data indicate that 72% of consumers rank taste as their top criterion when purchasing gummies. To address inherent mineral off-notes (e.g., the initial “fishy” note and bitter/astringent aftertaste of magnesium glycinate), RICHEN developed a flavor-reduced magnesium glycinate (Easy-Taste), removing key barriers to premium gummy formulation.
In a blind sensory evaluation with 50 participants (aqueous magnesium glycinate, 100 mg Mg/100 mL), RICHEN’s flavor-reduced magnesium glycinate exhibited a markedly weaker fishy note than competing brands and no discernible astringency, demonstrating a clear advantage in palatability.
Recommended gummy functional formula combination
|
Formula combination |
Core efficacy |
|
Zinc glycinate +Vitamin C |
Support healthy growth and development in children |
|
Ferrous glycinate + Vitamin C |
Correct iron deficiency efficiently and support hemoglobin synthesis |
|
Calcium glycinate + Vitamin D3 |
Regulate blood sugar and lipid levels and enhance bone metabolism |
|
Magnesium glycinate + Vitamin D3 + Vitamin B6 |
Support neural function, sleep quality, and emotional stability |
From global regulatory acceptance to application-level advances—and from enhanced absorption efficiency to optimized sensory performance—Pure-Chel™ glycine-chelated mineral salts are redefining development standards for mineral-fortified gummies. Leveraging RICHEN Nutritionals’ expertise in chelation technology, flavor-masking, and formulation synergy, brands can more efficiently address challenges in flavor, absorption, and product design, gaining a first-mover advantage in the rapidly expanding functional-gummy market.
[1] Hurrell, R., & Egli, I. (2010). Iron bioavailability and dietary reference values.The American Journal of Clinical Nutrition, 91(5), 1461S–1467S.DOI: 10.3945/ajcn.2010.28674F)
[2] Pineda O, Ashmead H D. Effectiveness of treatment of iron-deficiency anemia in infants and young children with ferrous bis-glycinate chelate[J]. Nutrition, 2001, 17(5): 381-384. DOI:10.1016/S0899-9007(01)00519-6.
[3] Cook J D, Reddy M B. Effect of ascorbic acid intake on nonheme-iron absorption from a complete diet[J]. Am J Clin Nutr, 2001, 73(1): 93-98. DOI:10.1093/ajcn/73.1.93.
[4] Wolff , N.A., Garrick, M.D., Zhao, L. et al. A role for divalent metal transporter (DMT1) in mitochondrial uptake of iron and manganese. Sci Rep 8, 211 (2018). https://doi.org/10.1038/s41598-017-18584-4
[5] Ganasen M, Togashi H, Takeda H, et al. Structural basis for promotion of duodenal iron absorption by enteric ferric reductase with ascorbate. Commun Biol. 2018;1:120. DOI:10.1038/s42003-018-0124-0
[6] Lenton K J, Therriault H, Cantin A M, et al. Direct effect of dietary ascorbate on intestinal iron oxidation state: an in vitro model[J]. Free Radical Biology and Medicine, 1999, 26(9-10): 1203-1208. DOI:10.1016/S0891-5849(98)00342-5.